
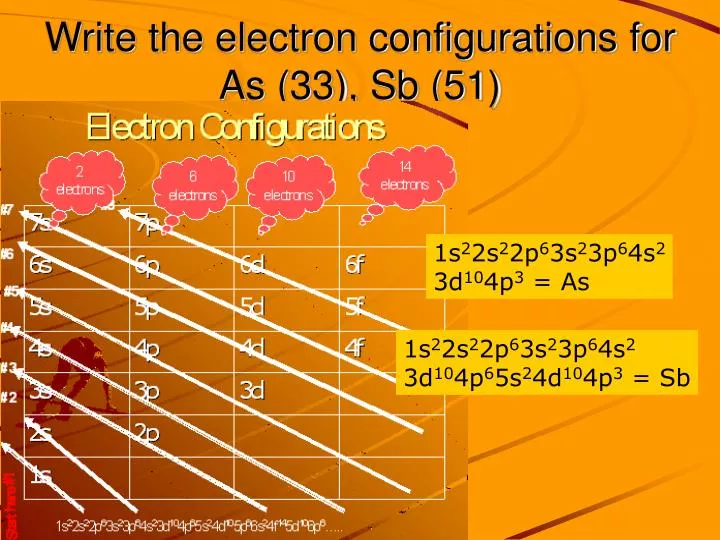
This element is also one of the heavier pnicogens but is very brittle and bad at conducting electricity. Antimony can either lose the three p electrons, resulting in a 3+ charge, or lose all five electrons to result in a 5+ charge. Furthermore, all pnictogens have five valence electrons two of these electrons are paired and exist in the s subshell, while the remaining three electrons exist in the p shell, unpaired. Pnictogens are special because they form strong double and triple covalent bonds (learn more about covalent bonds here) to produce stable compounds.Īntimony can react with almost all of the metals on the periodic table to form pnictides. Along with antimony, other elements of this group include nitrogen (learn about the discovery of nitrogen), phosphorus, arsenic, bismuth, and ununpentium. Pnictogen elements are members of the nitrogen group of the periodic table. Antimony has an electron configuration of 4d 105s 25p 3. It lies below arsenic, and above bismuth, and it has properties similar to both of those elements. It lies to the right of tin, and to the left of tellurium. One theory of Wolfgang Amadeus Mozart’s early death is that his doctor poisoned him with a toxic antimony medication.Īntimony, atomic symbol Sb, has atomic number 51 on the periodic table.Thankfully, people now know better to not follow these dangerous practices.

However, it has been widely used for different medicinal purposes, including laxatives.


 0 kommentar(er)
0 kommentar(er)
